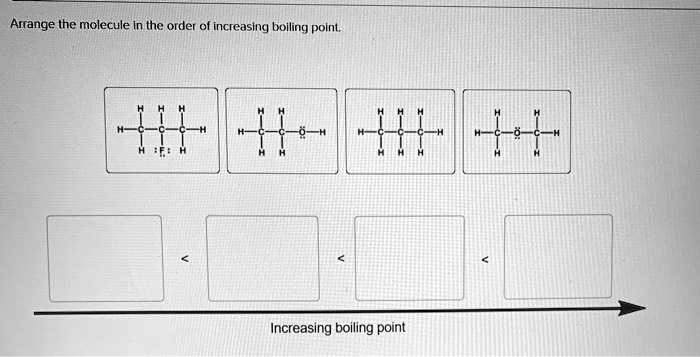
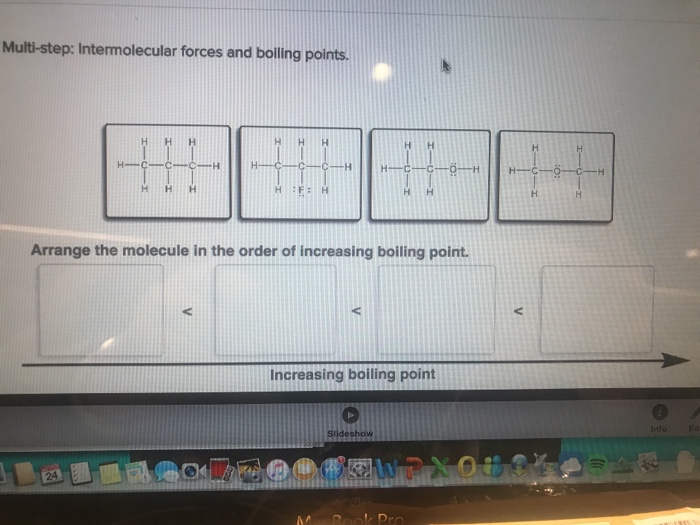
Arrange the molecule in the order of increasing boiling point. This concept forms the cornerstone of understanding the relationship between molecular structure and physical properties. By exploring the influence of molecular structure and intermolecular forces, we unravel the intricacies that govern the behavior of molecules and their boiling points.
Delving into the molecular realm, we discover how variations in molecular weight, polarity, and intermolecular forces shape the boiling points of substances. This knowledge equips us to predict and manipulate the properties of materials, paving the way for advancements in diverse fields.
Molecular Structure and Boiling Point: Arrange The Molecule In The Order Of Increasing Boiling Point.
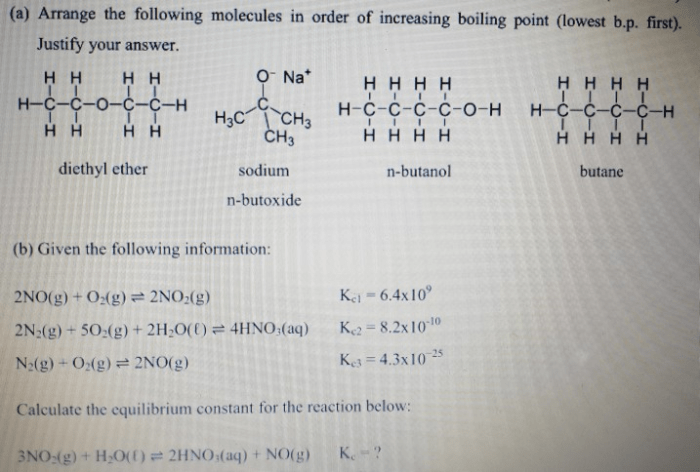
The molecular structure of a compound influences its boiling point. Molecules with larger surface areas and more complex shapes have stronger intermolecular forces and higher boiling points. For example, branched molecules have lower boiling points than linear molecules with the same molecular weight.
This is because the branched molecules have less surface area in contact with each other, resulting in weaker intermolecular forces.
Intermolecular Forces and Boiling Point
The strength of the intermolecular forces between molecules also affects their boiling point. Molecules with stronger intermolecular forces have higher boiling points. The types of intermolecular forces include:
- Hydrogen bonding
- Dipole-dipole interactions
- London dispersion forces
Hydrogen bonding is the strongest type of intermolecular force, followed by dipole-dipole interactions and then London dispersion forces.
Molecular Weight and Boiling Point, Arrange the molecule in the order of increasing boiling point.
In general, the molecular weight of a compound is directly proportional to its boiling point. This is because heavier molecules have more electrons and stronger intermolecular forces. However, there are exceptions to this rule, such as water, which has a higher boiling point than expected due to its strong hydrogen bonding.
Exceptions to the Trend
There are some exceptions to the general trend between molecular structure, intermolecular forces, and boiling point. For example, water has a higher boiling point than expected due to its strong hydrogen bonding. Another exception is ethanol, which has a lower boiling point than expected due to its ability to form hydrogen bonds with itself.
Quick FAQs
What factors influence the boiling point of a molecule?
Molecular structure, intermolecular forces, and molecular weight are the key factors that determine the boiling point of a molecule.
How does molecular structure affect boiling point?
Complex and branched molecular structures hinder intermolecular interactions, leading to higher boiling points. Conversely, compact and symmetrical molecules exhibit stronger intermolecular forces and lower boiling points.
What is the relationship between intermolecular forces and boiling point?
Stronger intermolecular forces, such as hydrogen bonding or dipole-dipole interactions, require more energy to overcome, resulting in higher boiling points. Weaker intermolecular forces, such as van der Waals forces, lead to lower boiling points.
How does molecular weight influence boiling point?
Generally, heavier molecules have higher boiling points due to their increased surface area and stronger intermolecular forces. However, this trend may vary based on molecular structure and intermolecular interactions.