Consider a hypothetical metal that has the following lattice parameters – Consider a hypothetical metal with precisely defined lattice parameters, embarking on an intriguing exploration of the intricate relationship between a material’s atomic arrangement and its macroscopic properties. This hypothetical metal serves as a captivating canvas upon which we paint a vivid picture of the profound influence lattice structure exerts on a material’s mechanical, electronic, thermal, and other characteristics.
Delving into the realm of crystallography, we unravel the principles of X-ray diffraction, a powerful technique that unveils the atomic architecture of materials. This knowledge empowers us to discern the diverse crystal systems and their unique signatures, laying the foundation for understanding how atomic arrangements govern material behavior.
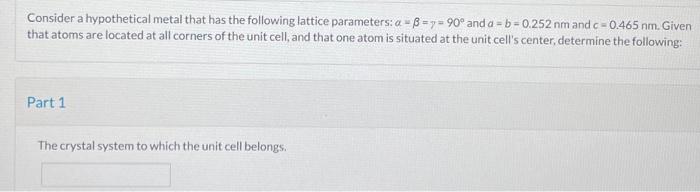
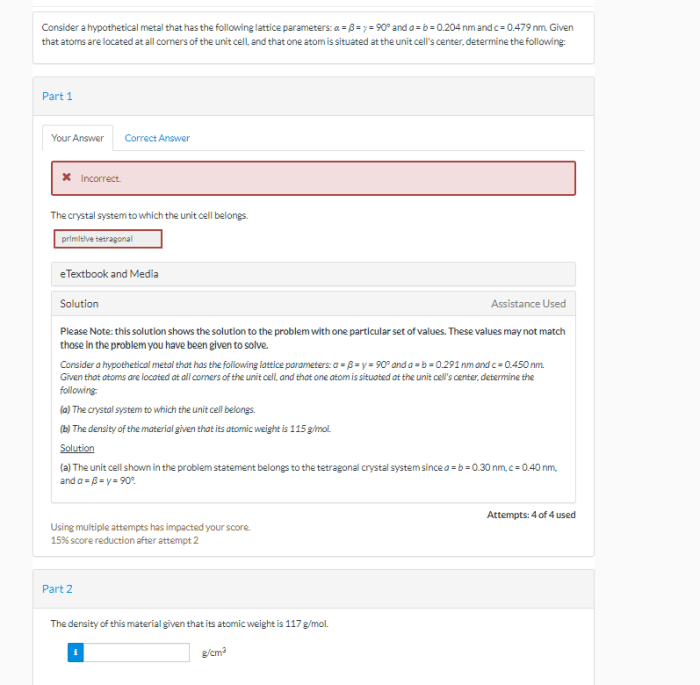
Lattice Structure
A lattice structure is a regular arrangement of atoms, molecules, or ions in a solid material. It is a fundamental concept in materials science as it determines the physical properties of the material. There are several types of lattice structures, including cubic, tetragonal, hexagonal, and monoclinic.
The lattice structure of a material is determined by the arrangement of its constituent particles. The most common lattice structures are cubic, tetragonal, and hexagonal. Cubic structures are characterized by a repeating pattern of cubes, tetragonal structures by a repeating pattern of rectangular prisms, and hexagonal structures by a repeating pattern of hexagons.
The lattice structure of a material has a significant influence on its physical properties. For example, cubic structures are typically strong and ductile, tetragonal structures are typically hard and brittle, and hexagonal structures are typically soft and malleable.
Crystallography
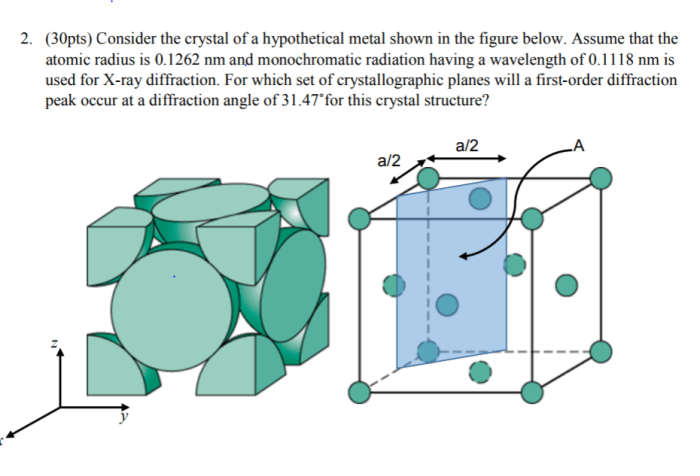
Crystallography is the study of the arrangement of atoms, molecules, or ions in a solid material. It is a branch of materials science that uses X-ray diffraction to determine the crystal structure of materials.
X-ray diffraction is a technique that uses X-rays to determine the arrangement of atoms in a crystal. X-rays are electromagnetic radiation with a wavelength that is comparable to the spacing between atoms in a crystal. When X-rays are shone on a crystal, they are diffracted by the atoms in the crystal.
The diffraction pattern can be used to determine the arrangement of atoms in the crystal.
Crystallography is a powerful tool that can be used to determine the crystal structure of a material. This information can be used to understand the physical properties of the material and to design new materials with specific properties.
Mechanical Properties
The mechanical properties of a material are its ability to withstand forces and deformations. These properties include strength, hardness, and toughness.
Strength is the ability of a material to resist deformation under an applied force. Hardness is the ability of a material to resist scratching or indentation. Toughness is the ability of a material to absorb energy without fracturing.
The mechanical properties of a material are determined by its lattice structure, the strength of the bonds between its atoms, and the presence of defects in the material.
Electronic Properties: Consider A Hypothetical Metal That Has The Following Lattice Parameters
The electronic properties of a material are its ability to conduct electricity and heat. These properties include electrical conductivity, thermal conductivity, and optical properties.
Electrical conductivity is the ability of a material to conduct electricity. Thermal conductivity is the ability of a material to conduct heat. Optical properties are the ability of a material to interact with light.
The electronic properties of a material are determined by its lattice structure, the number of electrons in its atoms, and the energy levels of its electrons.
Thermal Properties
The thermal properties of a material are its ability to store and conduct heat. These properties include specific heat capacity, thermal conductivity, and thermal expansion.
Specific heat capacity is the amount of heat required to raise the temperature of a material by one degree Celsius. Thermal conductivity is the ability of a material to conduct heat. Thermal expansion is the change in the volume of a material with temperature.
The thermal properties of a material are determined by its lattice structure, the strength of the bonds between its atoms, and the presence of defects in the material.
Applications
Materials with different lattice structures are used in a wide variety of applications. For example, cubic structures are used in metals, tetragonal structures are used in ceramics, and hexagonal structures are used in polymers.
The lattice structure of a material determines its physical properties, which in turn determines its suitability for different applications. For example, cubic structures are strong and ductile, making them suitable for use in load-bearing applications. Tetragonal structures are hard and brittle, making them suitable for use in cutting tools.
Hexagonal structures are soft and malleable, making them suitable for use in packaging and insulation.
Question Bank
What is the significance of lattice structure in materials science?
Lattice structure plays a pivotal role in determining the physical and chemical properties of materials. It influences strength, ductility, thermal conductivity, electrical conductivity, and other crucial characteristics.
How can we determine the lattice structure of a material?
X-ray diffraction is a widely used technique for determining the lattice structure of materials. By analyzing the diffraction patterns, scientists can identify the arrangement of atoms and molecules within the crystal structure.
How does lattice structure affect the mechanical properties of a material?
Lattice structure has a significant impact on the mechanical properties of a material. For instance, materials with a face-centered cubic (FCC) structure tend to be more ductile and malleable, while materials with a body-centered cubic (BCC) structure are generally stronger and harder.